In recent years, countries around the world, including my country, have strengthened the construction of drug traceability systems in order to combat counterfeit and shoddy drugs and improve drug safety supervision capabilities, and strive to promote the realization of drug traceability. The United States and the European Union have passed legislation requiring the addition of safety features to the outer packaging of drugs and the establishment of a drug traceability system. The DataMatrix code symbol on the drug packaging is widely used in the drug traceability process as a unique identifier that allows the authenticity of the drug to be verified. Therefore, the correct and compliant use of the DataMatrix code on the drug packaging is of great importance.
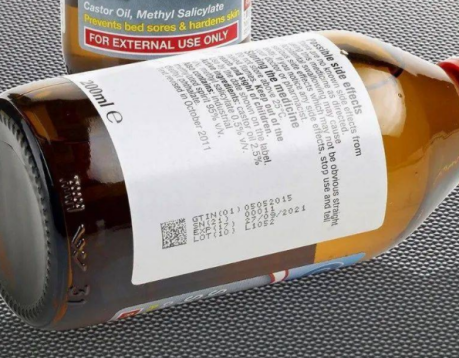
Enterprises using DataMatrix codes on drug packaging should pay attention to the following aspects:
(1) Choose GS1 standard. GS1 is a universal international standard system, which is widely used and followed by countries around the world in the global product traceability practice. It is the defacto international product traceability standard. Choosing GS1 to give products a globally unique product identification can enable product identification to obtain the most effective support and widest compatibility in the global supply chain, and effectively reduce the cost of traceability applications.
(2) The DataMatrix code used on the drug packaging is usually the GS1 DataMatrix code, not the traditional DataMatrix. The GS1 DataMatrix code is based on the DataMatrix ECC200, and the special character FNC1 is added to the front of the data, which makes it different from the traditional DataMatrix code and fully supports the GS1 standard. GS1 DataMatrix codes are widely used in the pharmaceutical industry in the United States and many European countries.
(3) The coding content of the DataMatrix code on the drug packaging should be determined according to the requirements of the specific application. Different application regions and different application environments have different requirements for coding content, usually including application indicator (AI), global trade item number (GTIN), production date (PROD DATE), expiration date ( EXPIRY), batch number (BATCH/LOT), serial number (SERIAL) and other information.
(4) The DataMatrix code is currently the smallest QR code in the world, which is very suitable for printing on pharmaceutical packaging with very limited printing area.
The size of the DataMatrix code on the drug package is usually determined by two factors:
(1) The amount of encoded content. The DataMatrix code generation system will automatically determine the symbol size specification and error correction level according to the encoded content, and generate the corresponding DataMatrix code. So in general, the more encoded content, the larger the size of the barcode.
(2) When the coding content is constant, the smaller the size of the unit modules that constitute the DataMatrix code, the smaller the corresponding DataMatrix code. However, the smaller the unit module, the more difficult it is to ensure the printing quality and reading efficiency of the two-dimensional code symbols. Therefore, in order to ensure the printing quality and reading efficiency of the two-dimensional code symbols, the unit module cannot be too small. GS1's recommendation on the size of the DataMatrix code unit module (X) is shown in Table 1.
DPM Code/X Dimension | Suggested Dimension | Max. Dimension | Min. Dimension |
DPM(ink) | 0.300mm | 0.615mm | 0.254mm |
DPM(Laser Engraved) | 0.200mm | 0.300mm | 0.100mm |
DPM(dot matrix) | 0.300mm | 0.495mm | 0.200mm |
After making the DataMatrix code, qualified enterprises can use professional QR code quality testing instruments or entrust a professional barcode quality testing agency to check the quality of barcode symbols in accordance with the current national standard GB/T 23704-2017 "2D Barcode". Inspection of the printing quality of barcode symbols" Carry out the quality inspection of DataMatrix code symbols to ensure that the symbol level is not lower than 1.5 (recommended level 2.5 or higher), so that the DataMatrix code can be correctly and efficiently read in the application environment.
Shenzhen Rakinda is specilized in barcode scanning for 22 years, and offers a series of barcode scanners for GS1 DataMatrix code reading. RK65 is one of the high-performance fixed mount industrial barcode scanner designed for production line automation applications.